Date: November 2022
Glioblastoma (GBM) patients are known to have a poor prognosis and tumor progression with high mortality and a median survival of only 12-15 months. Currently, anti-GBM therapy includes a multi-modality method involving radiotherapies and surgical removal of the tumor in combination with chemotherapy.1
Unfortunately, these tumors can often be resistant to radiotherapy as well as chemotherapy and surgical removal of the entire tumor may not be possible.1 New immunotherapy drugs may provide an alternate approach to treatment, aiming to bolster the immune system to eradicate disease. However, GBM is a highly complex disease with limited effective treatment options because of its highly angiogenic nature and its notorious ability to suppress the immune system both locally at the tumor and systemically in the body (Fig. 1).1,2 The brain being an immune-privileged tissue poses challenges in addition to the blood-brain barrier that further limits our therapeutic options such as the use of antibody therapies.1 However, investigation of immuno-oncology drugs may overcome this limitation as one may only need the immune cells to cross the blood-brain barrier. In addition, targeting the mechanisms by which glioma cells evade destruction by the immune system may allow us to provide patients with a meaningful survival benefit.2
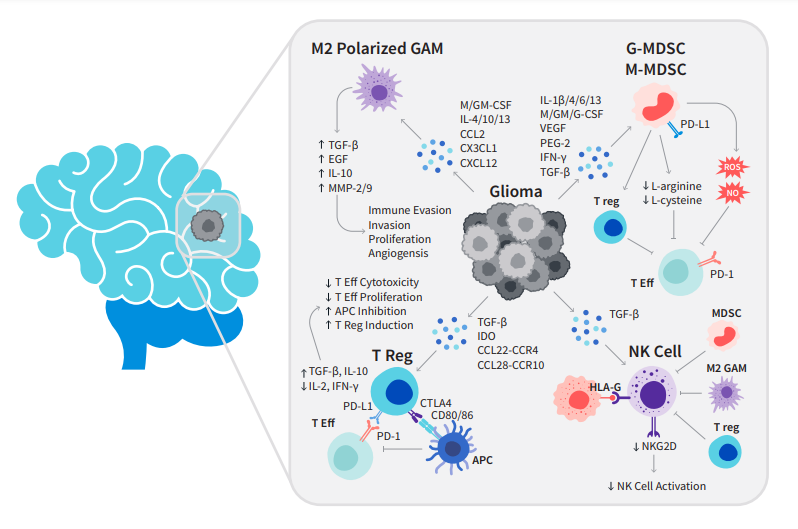
Figure 1: Interactions of the glioma with the immune system. Gliomas either have a direct inhibitory effect or attract/induce immunosuppressive cell types by expressing/secreting various factors. APC = antigen-presenting cell; GAM = glioma-associated microglia/macrophage; MDSC=myeloid-derived suppressor cell; MHC = major histocompatibility complex; NK = natural killer; NO = nitric oxide; ROS = reactive oxygen species; T Eff = effector T-cell; T reg = regulatory T-cell.
这类药物的临床前测试需要使用一组独特的临床前模型,这些模型采用具有免疫能力的小鼠以及将肿瘤细胞原位放置在脑内。 We have the technical capability to perform 60 orthotopic GBM procedures a day.
Orthotopic GBM Models |
D54-Luc Gli36-DsRed-R-Luc LN827 (pMMP-LucNeo) U251-Luc-mCH-Puro U87-MG-Luc |
人类 |
GL261-Luc2 C6* |
小鼠 |
|
9L* |
大鼠 |
*C6 and 9L are not Luc enabled. Luc-enabled models allow for longitudinal, in vivo insight into disease progression and responsiveness to treatment via bioluminescence imaging versus traditional survival endpoints alone.
GL261-Luc
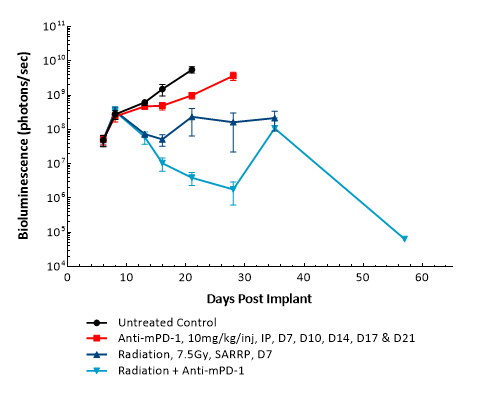
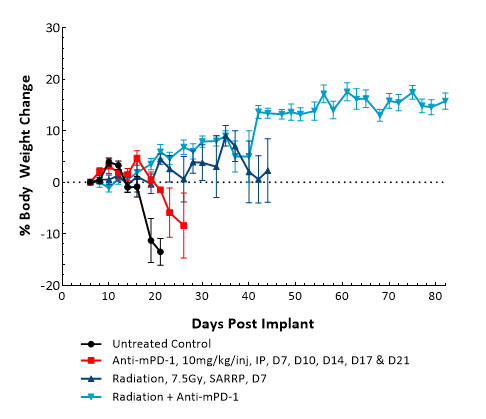
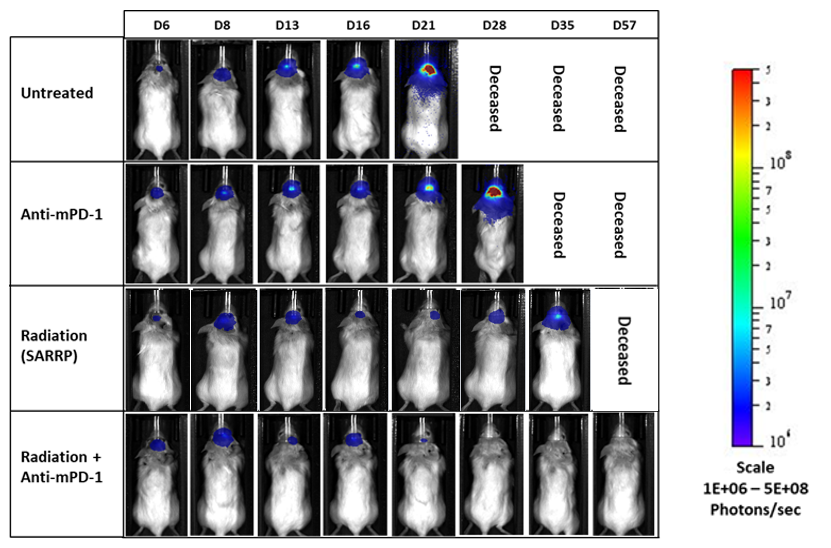
GL261是最常用的同基因鼠神经胶质瘤模型之一。 It was isolated from a C57BL/6 mouse with GBM. Intracranial (IC) implantation of this murine glioma cell line (1.00E+06 cells) in 6- to 7-week-old female C57BL/6 albino mice results in a fast-growing orthotopic GBM model. Based on the bioluminescence imaging (BLI) signal output, approximate staging duration is 6 days with a median tumor doubling time of ~2.7 days. In this syngeneic model, tumor progression is accompanied by body weight loss and most untreated animals do not survive past day 25. Combination of radiotherapy and anti-mPD-1 resulted in 87.5% tumor-free survivors (TFSs) (7/8). Focal radiation at 7.5 Gy alone had a moderate response with 25% TFSs (2/8), whereas anti-mPD-1 treatment alone elicited only a mild response. These results were found to be consistent in multiple studies at Labcorp. Further analyses of the immune profile and changes with treatment in different immune cell subsets have been done in this model using flow cytometry.
立即订阅
Stay up to date on the latest preclinical oncology advancements and Labcorp company news by subscribing to our emails.
D54-MG-Luc
D54 was isolated from a 53-year-old male patient with GBM. Implantation of this human glioma cell line (1.00E+05 cells) in 5- to 6-week-old female nude mice results in IC tumor growth that is slower than GL261-Luc with a staging duration of 12 days and a median tumor doubling time of ~3.6 days. In this model, treatment with cyclophosphamide (20 mg/kg) is well tolerated but fails to produce statistically significant tumor growth delay. Treatment with carmustine (BCNU) (15 mg/kg) demonstrated a statistically significant response and was well tolerated.
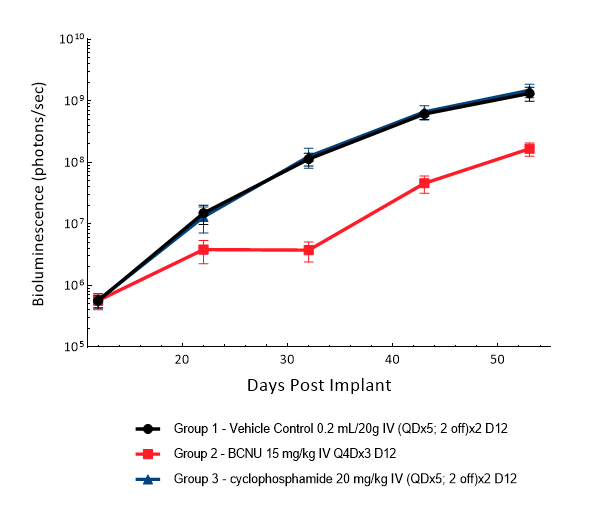
Figure 5: D54-MG-Luc IC model: mean tumor burden with standard error. IV = intravenous; Q4D = every 4 days; QD = once a day.
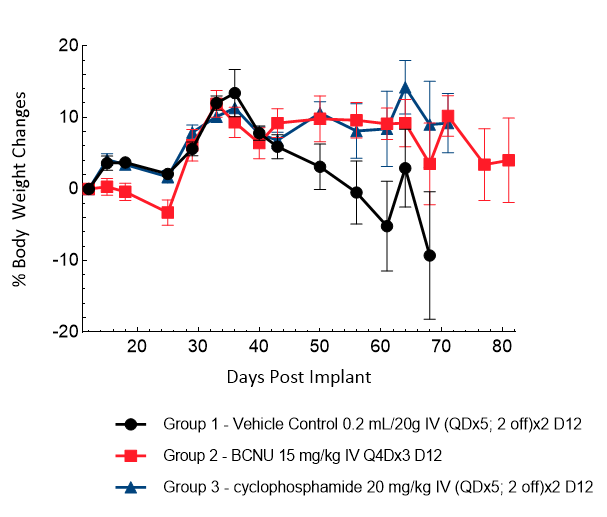
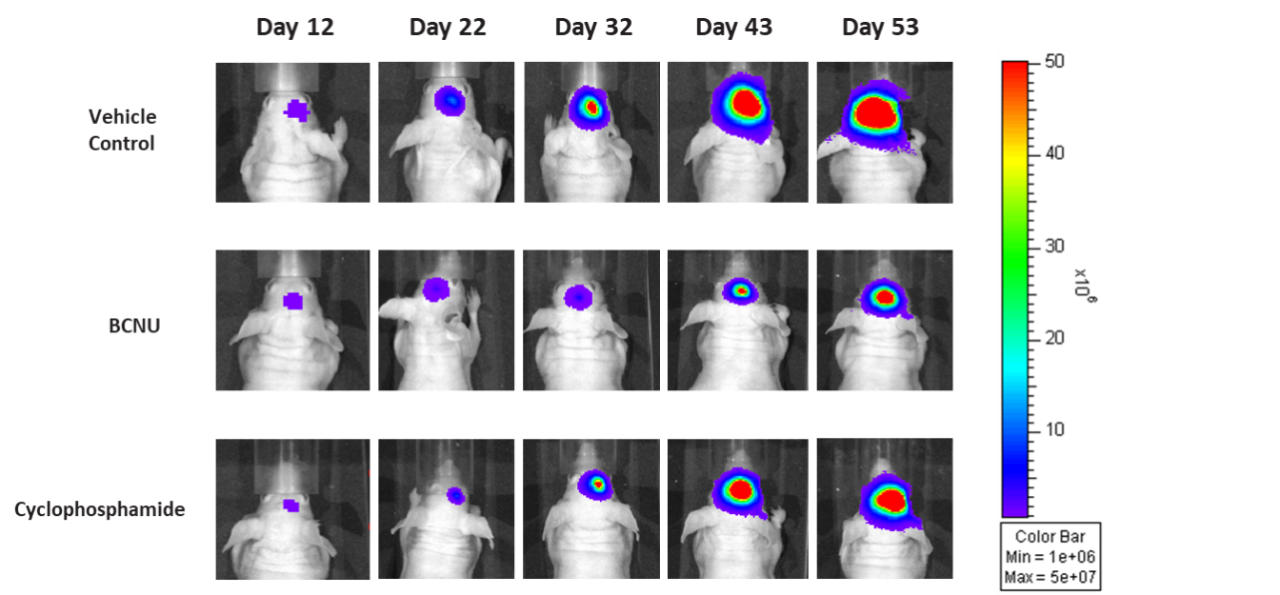
Figure 7: Representative images of BLI in each D54-MG-Luc implanted group showing localized signal in the brains of tumor-bearing mice. BLI signal intensity (photons/sec) is measured by the scale on the right.
Gli36-DsRed-R-luc (rescued)
Gli36 was isolated from a patient with GBM. Implantation of this human glioma cell line (5.00E+04 cells) in 5- to 6-week-old female C.B-17 severe combined immunodeficient (SCID) mice results in a fast-growing IC tumor with a staging duration of 6 days and a median tumor doubling time of ~3.3 days. In this model, treatment with temozolomide (TMZ) (100 mg/kg) is well tolerated and there is a statistically significant reduction in tumor burden as shown by the BLI output.
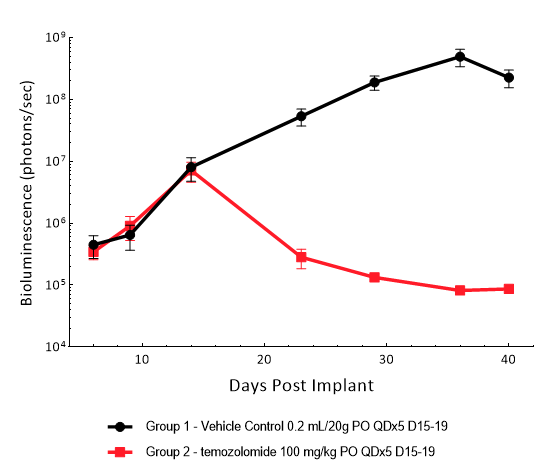
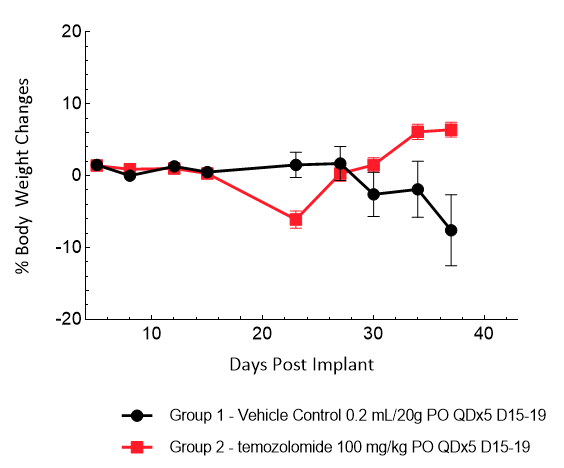
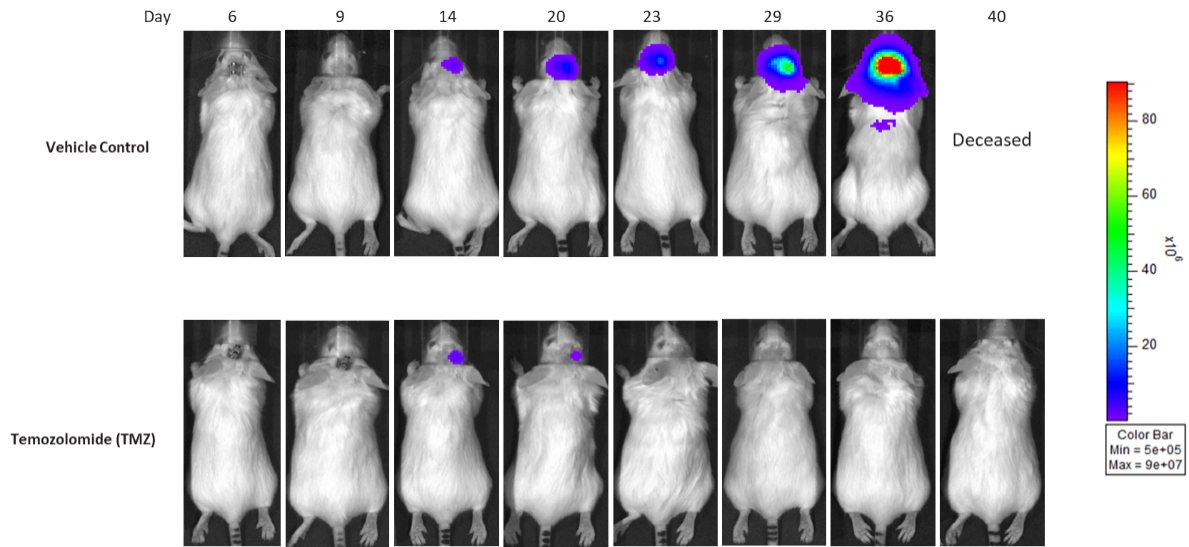
Figure 10: Representative images of BLI in each Gli36-DsRed-R-luc-implanted group showing localized signal in the brains of tumor-bearing mice. BLI signal intensity (photons/sec) is measured by the scale on the right.
LN827 (pMMP-LucNeo)
LN827 was isolated from a 72-year-old GBM patient. Implantation of this human glioma cell line (5.00E+04 cells) in 5- to 6-week-old female C.B-17 SCID mice results in an IC tumor growth that is a bit slower than the GL261-Luc with a staging duration of 8 days and a median tumor doubling time of ~2.9 days. In this orthotopic model, treatment with TMZ (100 mg/kg) is well tolerated and there is a statistically significant reduction in the tumor burden with time.
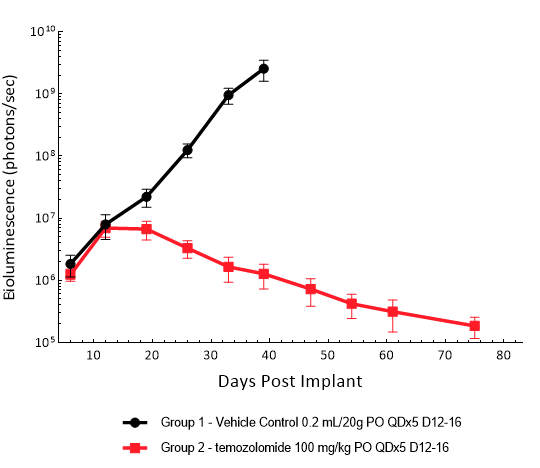
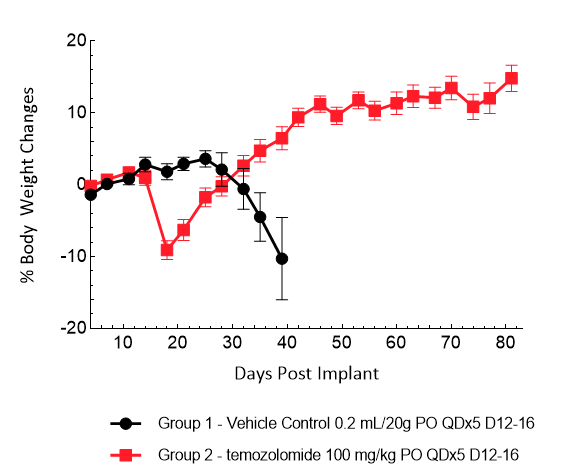
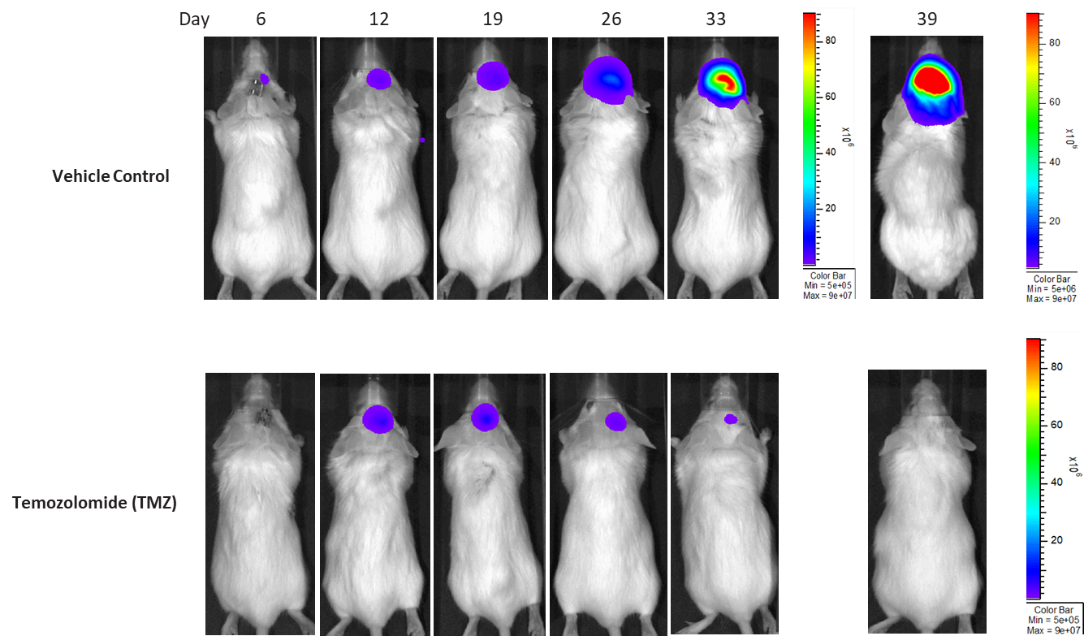
Figure 13: Representative images of BLI in each LN-827 implanted group showing localized signal in the brains of tumor-bearing mice. BLI signal intensity (photons/sec) is measured by the scale on the right.
U251-Luc-mCh-Puro
U251 was isolated from a 75-year-old male patient with astrocytoma. IC implantation of this human cell line (1.00E+06 cells) in 5- to 6-week-old female NSG and nude mice results in aggressive tumors. Based on the BLI signal output, approximate staging time is day 5 with a median tumor doubling time of ~ 2 days. In this model, treatment with TMZ (100 mg/kg) is well tolerated, reduces the tumor burden and improves overall survival.
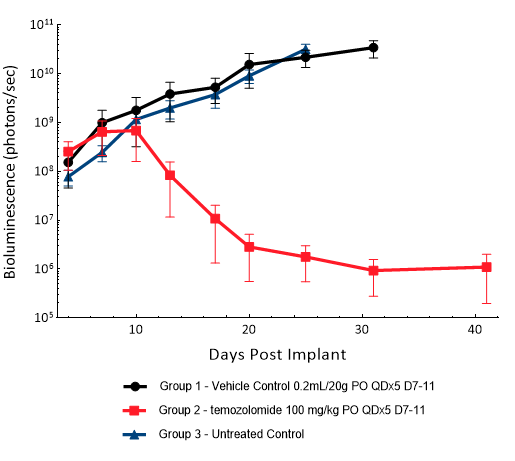
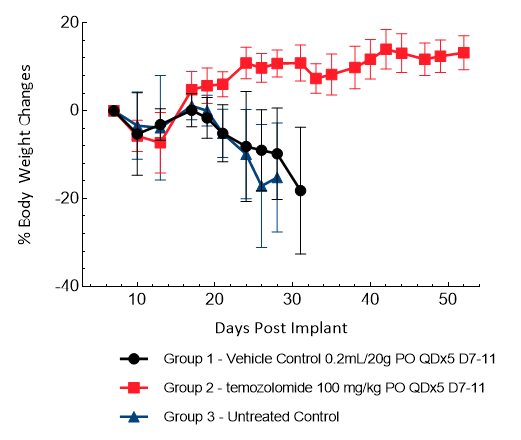
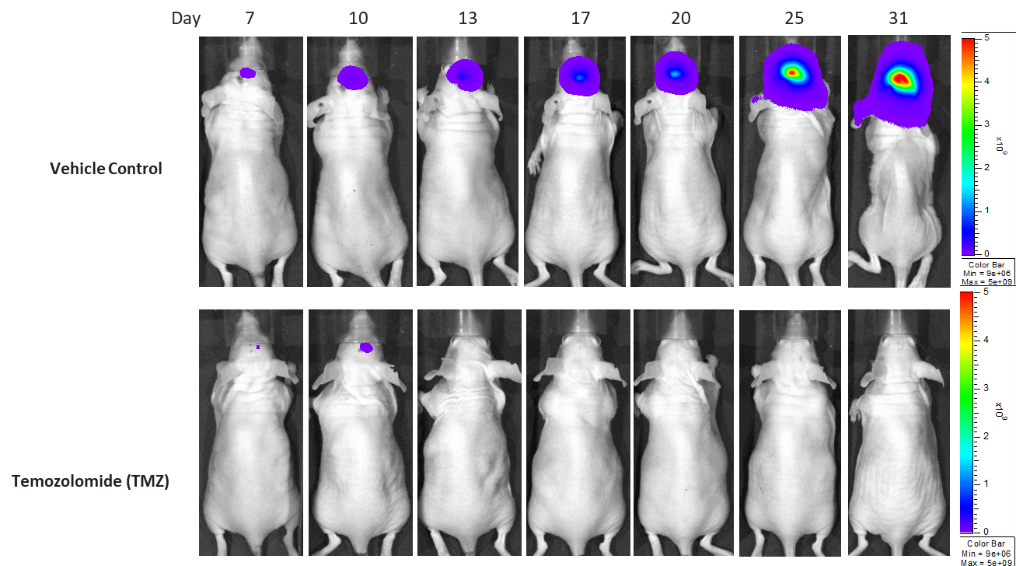
Figure 16: Representative images of BLI in each U-251-Luc-mCh-Puro implanted group showing localized signal in the brains of tumor-bearing mice. BLI signal intensity (photons/sec) is measured by the scale on the right.
U-87 MG-Luc
U87 was isolated from a 44-year-old female patient with GBM. IC implantation of this human cell line (1.00E+06 cells) in 6- to 7-week-old female nude mice results in consistent orthotopic tumor growth. Based on the BLI signal output, approximate staging time is day 21 with a median tumor doubling time of 4-5 days. In this model, disease progression is accompanied by body weight loss, hunched posture and a domed cranium with a median survival of ~48 days.
Treatment with sunitinib or anti-VEGFR2 antibody (DC101) does not have any meaningful response in this model. TMZ (100 mg/kg) treatment results in decreased brain BLI signal (5/6), complete remission and TFSs (4/6). Low doses of TMZ result in moderate responses with partial regression at 8 mg/kg (100%), 4 mg/kg (86%) and 2 mg/kg (43%). TMZ treatment does not result in additional body weight loss. Monotherapy with low-dose fractioned focal radiation (once a day x5) delivered by the SARRP produces anti-tumor responses with 15% TFSs (1/7) at 2 Gy radiation.
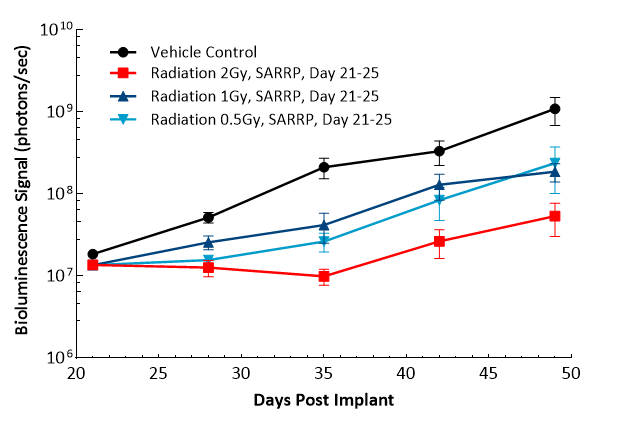
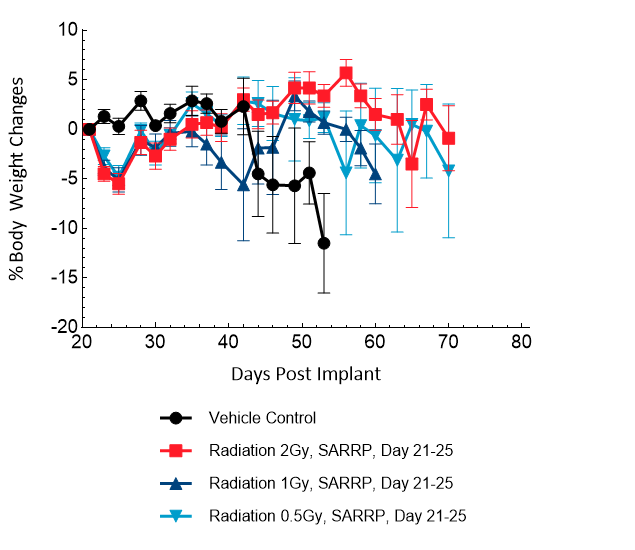
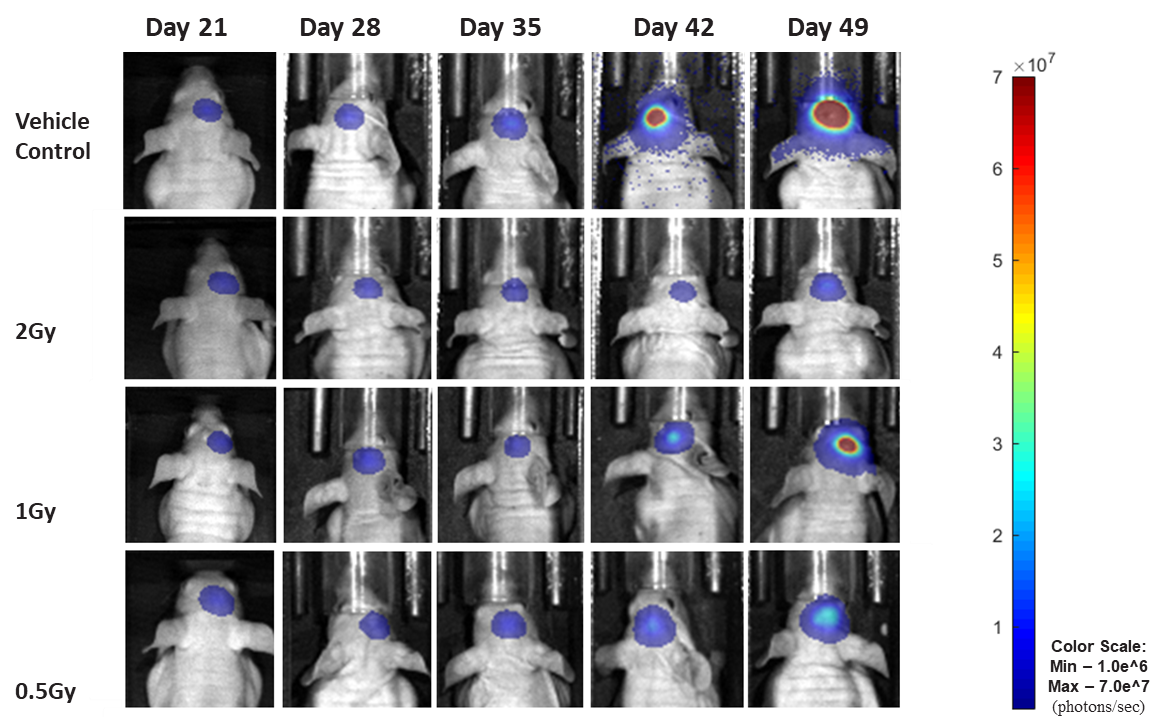
Figure 19: Representative images of BLI in each U-87 MG-Luc implanted group showing localized signal in the brains of tumor-bearing mice. BLI signal intensity (photons/sec) is measured by the scale on the right.
As in the clinical response to standard of care, preclinical treatment response is highly dependent on the tumor line being used. 但是,观察同一适应症的多种不同肿瘤系可以提供有关新疗法临床功效的关键信息。
At Labcorp, we offer a combination of multiple syngeneic tumor lines along with in vivo imaging, guided irradiation treatment, flow cytometry and nCounter® PanCancer IO 360™ panel analysis to best evaluate novel immunotherapies. Our high-throughput capabilities allow rapid and automated acquisition of large sample sizes to accommodate the growing complexity of our clients’ preclinical studies.
Please contact us to speak with our scientists about orthotopic glioblastoma models.
参考资料
1. Thakur A, Faujdar C, Sharma R, et al. Glioblastoma: current status, emerging targets, and recent advances. J Med Chem. 2022;65(13):8596-8685. doi:10.1021/acs.jmedchem.1c01946.
2. Grabowski MM, Sankey EW, Ryan KJ, et al. Immune suppression in gliomas. J Neurooncol. 2021;151(1):3-12. doi:10.1007/s11060-020-03483-y.
让我们开始对话
联系我们