Advance your ocular therapies with the only full-service contract research organization to offer personalized ophthalmic drug development services that span the entire continuum from discovery and preclinical to clinical trials and post-market follow-up studies.
Whether you need support with anterior or posterior segments, rare ocular diseases, gene therapies or ophthalmic devices and diagnostics, we know how to optimize your ophthalmic development.
为您的眼科计划制定战略愿景
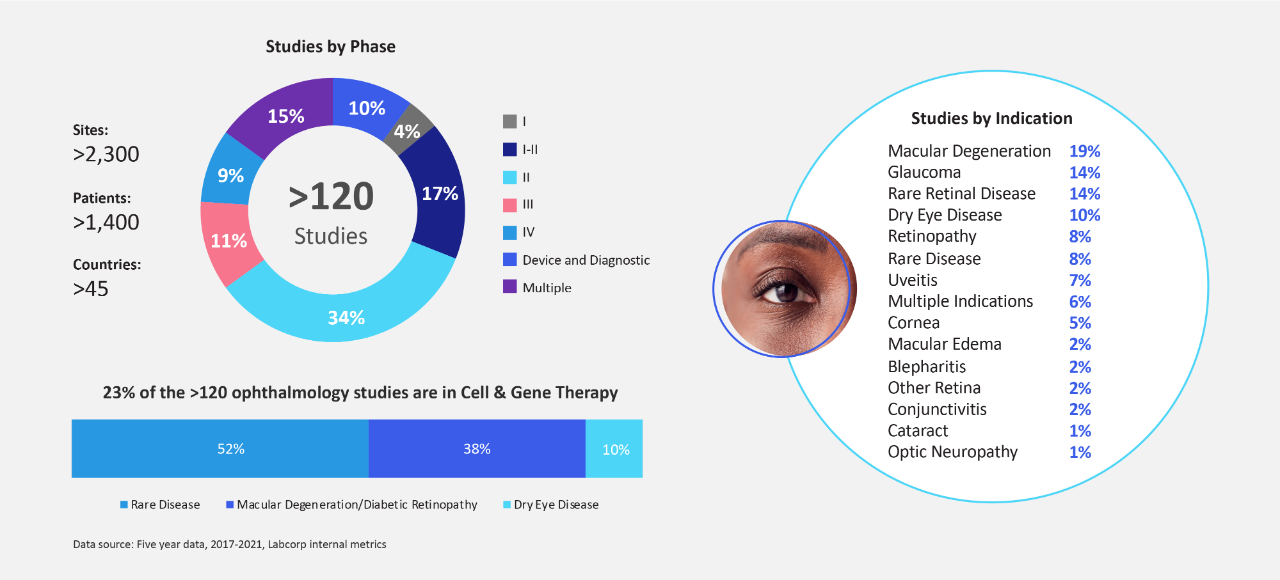
过去五年来,我们为新兴生物技术公司进行了超过120项以患者为中心的全球眼科临床试验,我们的眼科工作人员从中积累了丰富的科学和法规经验
Work with renowned vision scientists as part of our more than 30-year relationship with Ocular Services on Demand (OSOD) to handle your preclinical ocular needs and deliver specialized expertise in the ocular surface and anterior and posterior segments
Enable intelligent patient recruitment with advanced data and increase your efficiencies with our established relationships with more than 2,000 global sites
Extend your ophthalmic team’s expertise with a full-service collaboration
在决策时将患者的需求放在首位并确保您的眼科药物研发计划成功的方面,经验至关重要。在过去五年中,徕博科运用专业知识和见解帮助推进了120多项临床眼科研究,为新兴和中型生物技术公司提供灵活的解决方案。
获取所需的跨职能支持和定制策略,以加快研发进度、预测和预防问题并将眼科疗法推向市场。与我们建立强大的合作伙伴关系,帮助您的团队更快地改善患者生活。
获取量身定制的临床前解决方案
You may need toxicology (good laboratory practices and non-good laboratory practices), tissue distribution/pharmacokinetics and pharmacology to support your ophthalmology preclinical research. 我们了解眼表、前段和后段之间的独特差异,并可以提供多个眼病领域的专业知识,例如青光眼、干眼、葡萄膜炎、过敏和视网膜疾病。
我们在所有药物/疗法类别的经验以及我们与OSOD的合作伙伴关系也可以让您的临床前试验受益,从而为多种模型提供眼部给药途径。
与徕博科一起进行眼科研究时,您的团队还可以使用我们的全方位服务实验室,以满足化学或生物分析服务方面的任何需求。探索我们一体式综合实力的与众不同之处。
Unique solutions for clinical trial testing
我们的交付成果以独特的临床试验和测试解决方案为中心,这些解决方案既以患者为本,又可以灵活地满足您方案的需求。
- Gain scientific and regulatory support – Extend your team's capabilities with our regulatory and commercial guidance. Count on our extensive experience in assisting both small biotech and large pharmaceutical companies to help you achieve your strategic goals
- Apply flexible, integrated delivery models – Whether you need to apply decentralized clinical trial approaches to mitigate delivery risks, we can work together to optimize your delivery
我们根据您的喜好和项目需求设计交付能力。